Abstract
Background:
Selection of first line treatment in patients with chronic myeloid leukemia (CML) is one of the major challenges in routine practice. While a wealth of comparative clinical and efficacy data are available for the three tyrosine kinase inhibitors (TKIs) that can be used frontline, there is dearth of information on their effects on patients' health-related quality of life (HRQOL).
Aim:
The primary objective was to investigate HRQOL differences in patients receiving first line therapy with imatinib (IMA) versus those receiving therapy with dasatinib (DASA) in the real-world setting. The pre-specified primary endpoint of the protocol was the "impact on daily life scale" of the EORTC QLQ-CML24 questionnaire.
Patients and methods:
This was a matched case control study recruiting patients from 38 centers in Italy and Germany. Patients selection criteria included: being in first line treatment with IMA or DASA for no more than three years and, at least, in complete cytogenetic response at the time of study entry. All patients were invited by their treating physicians in the hospital to participate and all consenting patients were requested to complete a Health Survey Packet at home. Pre-paid reply envelopes were also provided with the request to send back completed Surveys to an independent coordinating Data Center. HRQOL was assessed with the EORTC QLQ-C30 and the CML disease specific EORTC QLQ-CML24. This latter questionnaire was specifically developed for CML patients and includes the following scales: 1) impact on daily life; 2) impact on worry/mood; 3) body image problems; 4) satisfaction with care and information; 5) satisfaction with social life; 6) symptom burden. In order to minimize selection bias when performing HRQOL comparisons between treatment groups, IMA patients were matched to DASA patients by 1:1 optimal pair propensity score (PS) matching. By such procedure, we identified the most similar IMA-DASA pairs as based on patients' characteristics at diagnosis: age, sex, family status, comorbidity, ECOG performance status, Sokal risk and previous treatment for CML. In addition, we further adjusted comparisons between matched treatment groups by potential post-diagnosis HRQOL confounders, including months from treatment start, any adverse event that led to drug interruption and taking drug(s) for other concurrent disease(s). Only patients for whom both drugs were available at time of diagnosis were considered for the matching procedures.
Results:
Between October 2014 and December 2016, 323 patients, of whom 223 in therapy with IMA and 100 in therapy with DASA were consecutively enrolled. Patients' compliance was 97% of patients (N=312/323) returning a valid Health Survey Packet to the coordinating Data center. At study participation, mean age of patients was 65 and 60 years for patients treated with IMA and DASA respectively. Median time of therapy was 1.3 years for both IMA and DASA. All results reported below derive from adjusted comparisons between the PS-matched IMA-DASA groups (94 patients each).
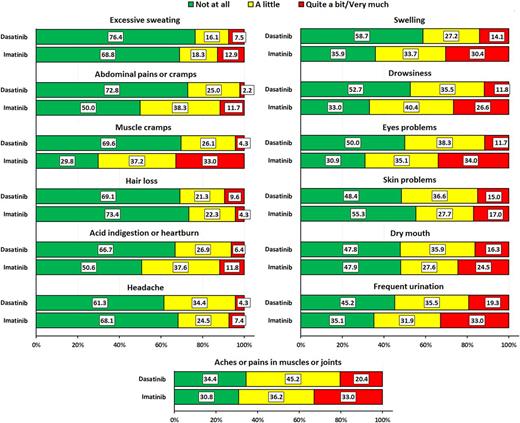
Analysis of the primary endpoint revealed that IMA patients had a statistically significant higher score (i.e., worse outcome) on the impact on daily life scale (P=.005). Other two scales of the EORTC QLQ-CML24 were significantly better for patients treated with DASA, that is, satisfaction with social life (P=.001) and symptom burden (P=.001). For descriptive purposes, CML symptom prevalence of those included in the EORTC QLQ-CML24 symptom burden scale is depicted in Figure 1. Conversely, there were no apparent better outcomes for patients treated with DASA for most of the scales of the cancer generic EORTC QLQ-C30 questionnaire (data not shown). Inspection of cancer generic symptoms revealed that pain (P=.013) and diarrhea (P=.016) were better for patients treated with DASA while constipation was better for patients treated with IMA (P=.026).
Conclusions:
This study suggests that patients in treatment with first line DASA report a lower impact of therapy on their daily life, compared to their peers in treatment with IMA. Some benefits in terms of symptom burden, favoring patients treated with DASA, were also noted as measured by a CML disease specific HRQOL questionnaire. This information extends current knowledge on physician-reported safety and efficacy data of IMA and DASA and might help clinicians making more informed treatment decisions.
Efficace: Seattle Genetics: Consultancy; TEVA: Consultancy, Research Funding; Incyte: Consultancy; Lundbeck: Research Funding; Amgen: Consultancy, Research Funding. Baccarani: Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Iurlo: Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Abruzzese: Bristol Myers Squibb: Consultancy; ARIAD: Consultancy; Incyte: Consultancy; Pfizer: Consultancy; Novartis: Consultancy. Breccia: TEVA: Speakers Bureau. Castagnetti: Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Crugnola: Novartis: Honoraria; Celgene: Honoraria; BMS: Honoraria. Tiribelli: Bristol Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria. Gaidano: Gilead: Consultancy, Honoraria; Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Bosi: Janssen: Honoraria; Celgene: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria. Pregno: Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Saussele: Incyte: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Rosti: Pfizer: Research Funding, Speakers Bureau; Bristol Myers Squibb: Research Funding, Speakers Bureau; Incyte: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal